HF EtO air cleaner
Ethylene oxide (EtO) is a harmful gas that is widely used for sterilizing medical products. During storage, it can continue to be released for months from the cardboard packaging and from underneath the plastic film. This release happens gradually, and because the gas is invisible and has a high odor threshold, concentrations in warehouses can build up significantly.


Step-by-step plan for a safe working environment
No two situations are alike when it comes to EtO. Every work environment has its own challenges, risks, and objectives. That’s why we guide companies step by step toward a structurally safe working environment, in close collaboration with a network of independent experts. This process is tailored to your specific needs and consists of four key phases.

Quick scan
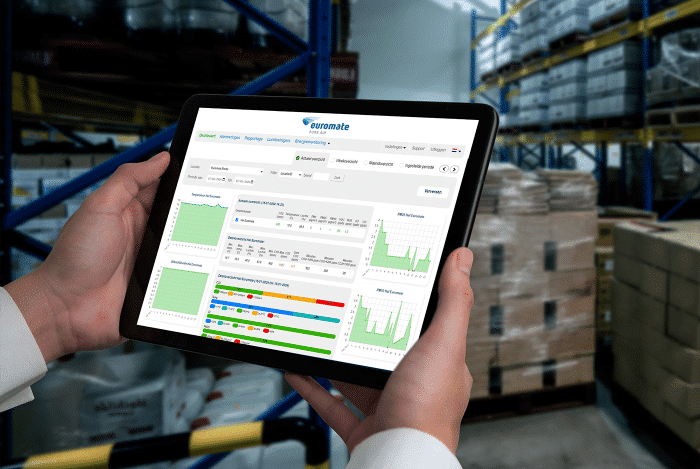
We start with an exploratory visit to assess the situation. Based on observations, we identify potential risk zones and determine where measurements will be most meaningful.

Detailed measurements
Actual EtO concentrations are measured in this phase. The data provides the foundation for analysis, identifying peaks, dispersion, and trends over time.

Risk assessment (RI&E) considerations and definition of ambitions
The measurement results are translated into concrete objectives. Together, we establish realistic reduction targets and priorities, aligned with legal standards and your business goals.

Detailed design and implementation plan
Based on measurements and objectives, we design a customized filtration and monitoring solution. We also advise on maintenance, periodic validation, and continuous improvement in line with the ALARA principle.
Would you like to learn more about the HF EtO air cleaner?
In our brochure, you can read more about its applications and how it works. After completing the form, you will receive the requested documentation in your inbox within a few minutes.
See all our products

DFI Series industrial air cleaners
- Ensures dust reduction of up to 90%
- customised solution based on dust concentration
- Suitable for warehouses, industrial and logistic halls

Pure Air Shield 3300
- Removes fine dust, viruses and bacteria
- Suitable for large spaces (from 200m2)
- SGS certified, proven effective

HF-Series Industrial air cleaners
- Filters up to 90% of the contamination
- Suitable for industrial environments
- Customized solution for different pollution sources

SF-Series Kitchen Air Filtration
- Filters more than 90% grease and soot particles from the air
- Ensures less risk of fire
- Total solution for every kitchen air problem

Smoke 'n Go
- Leaves no traces of tobacco smoke and unpleasant odours
- Five-stage filtration including HEPA or electrostatic filter
- Tested and certified by BGIA, TUV, ECN

VisionAir Blue Line
- Wide range of applications thanks to unique filter combinations
- SGS certified and therefore proven effective
- Has a positive effect on productivity and absenteeism

HF EtO air filtration
- Proven effective removal of ethylene oxide
- Contributes to a healthy and safe working environment
- Specially developed for logistics halls and warehouses
Would you like to know if EtO filtration is the right solution for your organization?